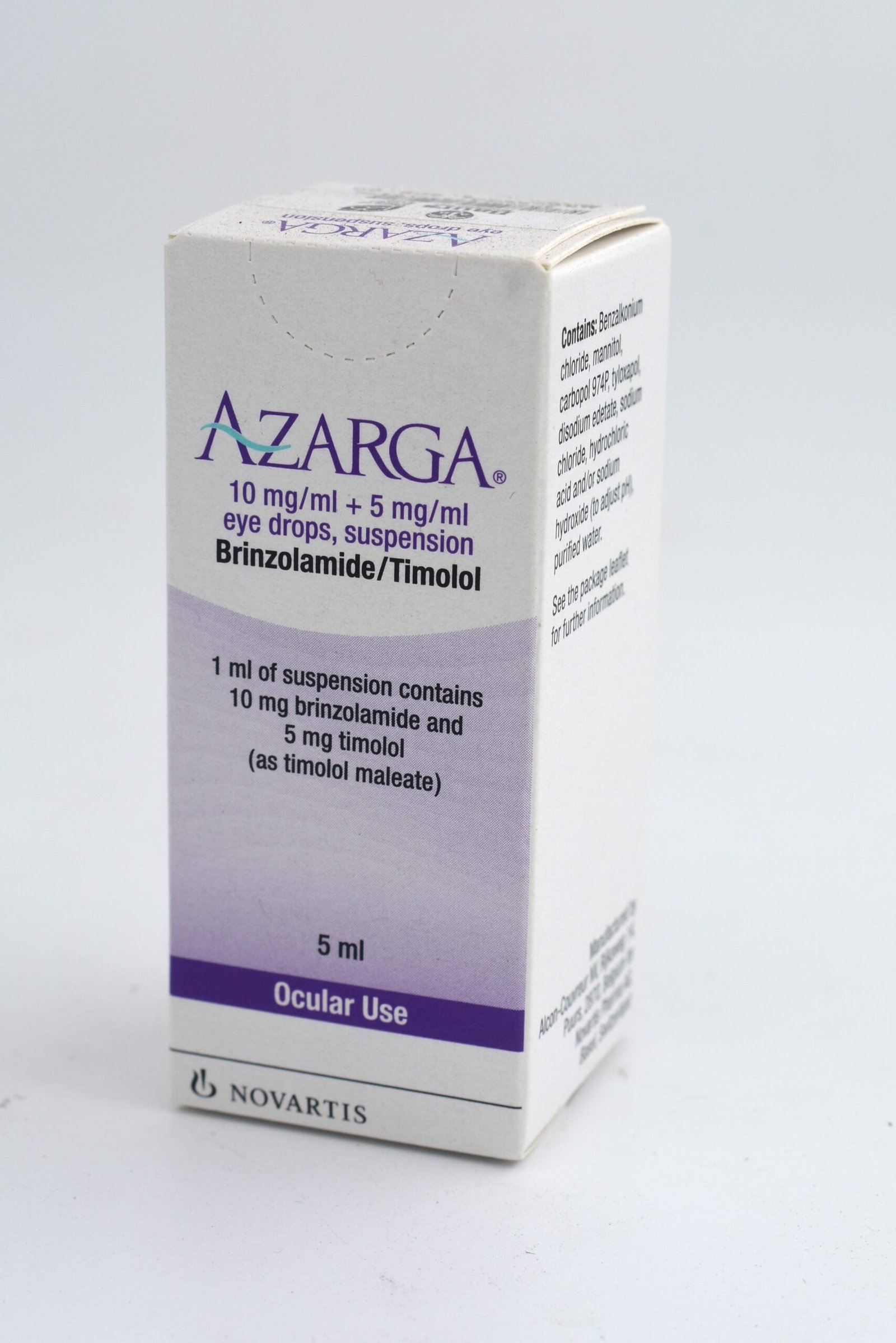
AZARGA 10 mg/ml + 5 mg/ml eye drops, suspension
One ml of suspension contains 10 mg brinzolamide and 5 mg timolol (as timolol maleate).
Excipient with known effect:
One ml of suspension contains 0.10 mg benzalkonium chloride.
Therapeutic indications
Decrease of intraocular pressure (IOP) in adult patients with open-angle glaucoma or ocular hypertension for whom monotherapy provides insufficient IOP reduction.
Posology and method of administration
Posology
Use in adults, including the elderly
The dose is one drop of AZARGA in the conjunctival sac of the affected eye(s) twice daily.
When using nasolacrimal occlusion or closing the eyelids, the systemic absorption is reduced. This may result in a decrease in systemic side effects and an increase in local activity (see section 4.4).
If a dose is missed, treatment should be continued with the next dose as planned. The dose should not exceed one drop in the affected eye (s) twice daily.
When substituting another ophthalmic antiglaucoma medicinal product with AZARGA, the other medicinal product should be discontinued and AZARGA should be started the following day.
Special populations
Paediatric population
The safety and efficacy of AZARGA in children and adolescents aged 0 to 18 years have not yet been established. No data are available.